
The Prescription Drug User Fee Act (PDUFA) action date is July 7, 2022. An updated label would allow the Company’s commercial team to promote KRYSTEXXA plus methotrexate to physicians.
#HORIZON THERAPEUTICS PLC EARNINGS TRIAL#
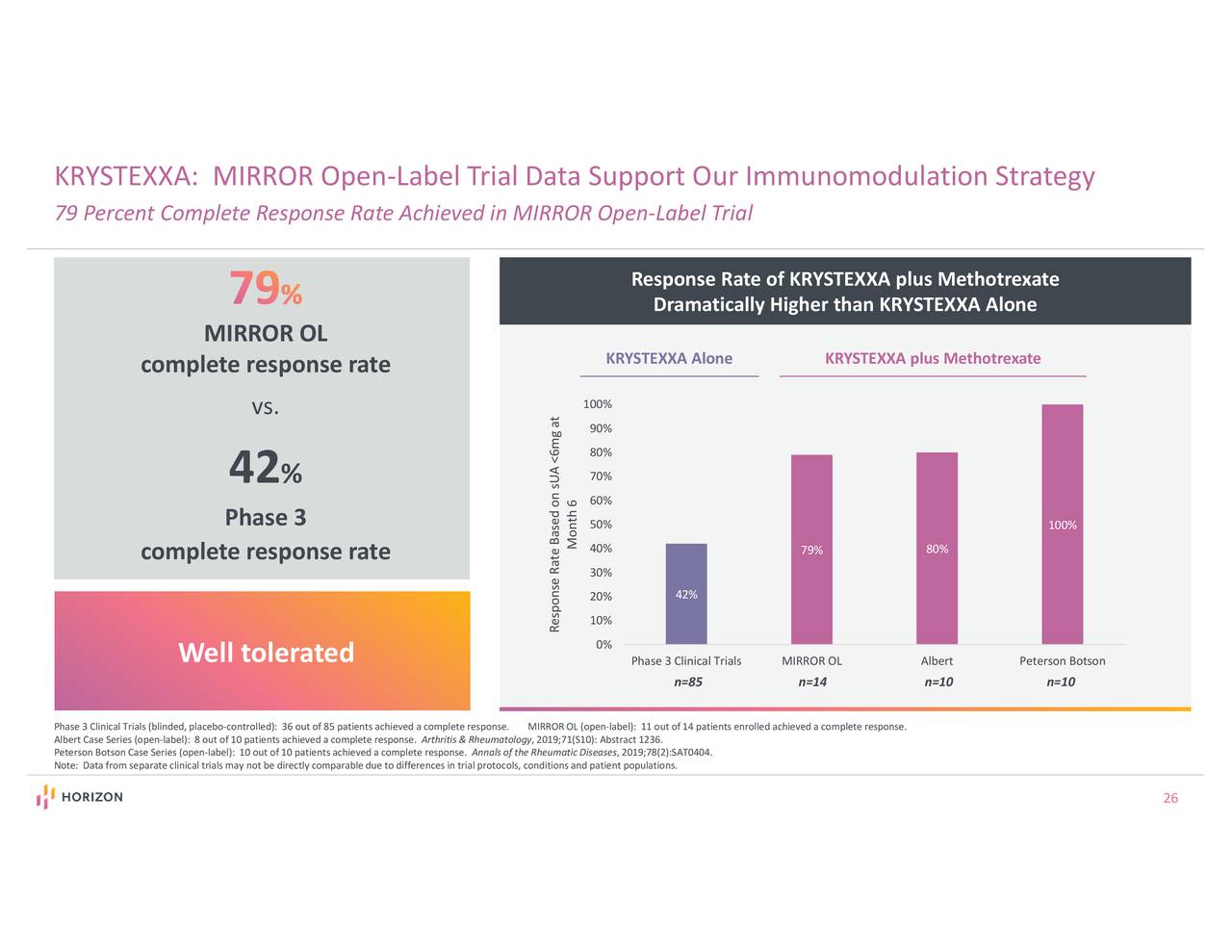
The Company submitted an sBLA in January based on results from the MIRROR Phase 4 randomized placebo-controlled trial which demonstrated that 71% of patients receiving KRYSTEXXA plus methotrexate achieved a complete response, a more than 30 percentage-point improvement compared to patients who were randomized to receive KRYSTEXXA plus placebo (p<0.0001). Food and Drug Administration (FDA) granted priority review of the Company’s sBLA to expand the label for KRYSTEXXA to include co-treatment with methotrexate. FDA Granted Priority Review of sBLA for Co-Treatment of KRYSTEXXA Plus Methotrexate: In March, the U.S.government-mandated COVID 19-vaccine orders.įirst Quarter and Recent Company Highlights Announced Positive Topline Data from Phase 2 Trial Evaluating Dazodalibep (HZN-4920) in Patients with Rheumatoid Arthritis (RA) Study Met Primary Endpoint and Dazodalibep was Well Tolerated -įirst-quarter 2021 results were negatively impacted by a short-term TEPEZZA supply disruption due to U.S.
Initiated Two Clinical Trials to Date, Five Additional Trials Expected to Initiate this Year. Initiated Launch Preparations to Support Potential Approvals for TEPEZZA and UPLIZNA in Brazil as part of Global Expansion Strategy. European Commission Approved UPLIZNA ® (inebilizumab-cdon) for the Treatment of Adult Patients with NMOSD.
#HORIZON THERAPEUTICS PLC EARNINGS LICENSE#
FDA Granted Priority Review of the Company’s Supplemental Biologics License Application (sBLA) for Co-Treatment of KRYSTEXXA Plus Methotrexate with a JPDUFA Action Date. Continue to Expect KRYSTEXXA Net Sales Growth of More Than 20%. Continue to Expect TEPEZZA Net Sales Percentage Growth in the Mid-30s. Maintaining Full-Year 2022 Adjusted EBITDA Guidance of $1.63 Billion to $1.70 Billion, Representing 30% Growth and 230 Basis Points of Margin Expansion at the Midpoint.
Maintaining Full-Year 2022 Net Sales Guidance of $3.9 Billion to $4.0 Billion, Representing 22% Growth at the Midpoint. Cash Position of $1.64 Billion as of Ma.

KRYSTEXXA ® (pegloticase injection) Net Sales of $140.7 Million. TEPEZZA ® (teprotumumab-trbw) Net Sales of $501.5 Million. GAAP Net Income of $204.3 Million Adjusted EBITDA of $371.2 Million.


 0 kommentar(er)
0 kommentar(er)
